FDA Drug Recall Alert – UPDATE – Cefazolin for Injection, USP, 1 gm vial
| Date: | July 17, 2025 |
| From: | Health Plan of San Joaquin/Mountain Valley Health Plan (“Health Plan”) |
| To: | Health Plan Practitioners, Facilities, and Hospitals |
| Type: | Informational/Educational |
| Subject: | FDA Drug Recall Alert – UPDATE – Cefazolin for Injection, USP, 1 gm vial |
| Business: | Medi-Cal Managed Care |
On July 15, 2025, the Food and Drug Administration (FDA) released a recall announcement for Cefazolin for Injection, USP, 1 gm vial.
This is an update to the company statement issued on June 27, 2025.
This is for informational purposes only. You may or may not have administered the medication. Please disregard if you have not been affected by this recall.
For the complete details regarding this recall announcement, please visit the following web link: Update – Sandoz Inc. Issues Voluntary Nationwide Recall Expansion of One Additional Lot of Cefazolin for Injection Due to Vials Being Potentially Mislabeled as Penicillin G Potassium for Injection | FDA
Update – Sandoz Inc. Issues Voluntary Nationwide Recall Expansion of One Additional Lot of Cefazolin for Injection Due to Vials Being Potentially Mislabeled as Penicillin G Potassium for Injection
Summary
Company Announcement Date:
July 15, 2025
FDA Publish Date:
July 15, 2025
Reason for Announcement:
Vials incorrectly labelled as Penicillin G Potassium for Injection contain Cefazolin for Injection
Company Name:
Sandoz, Inc.
Brand Name:
Sandoz
Product Description:
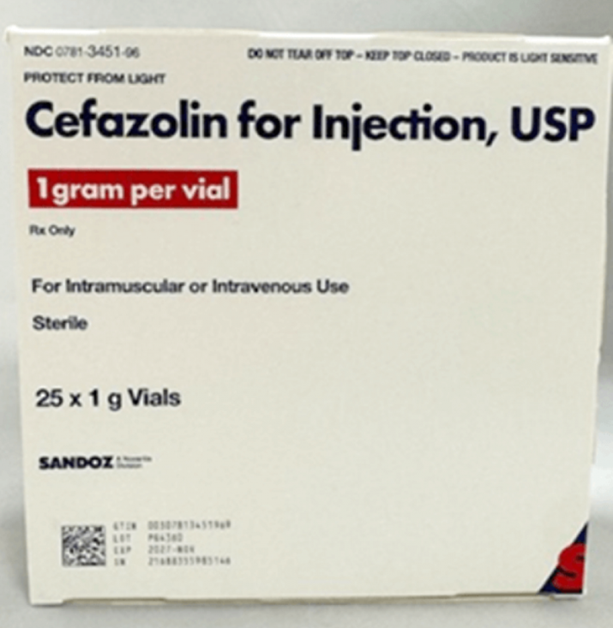
Cefazolin for Injection, USP, 1 gm vial
Company Announcement
This is an update to the Company Statement issued on June 27, 2025.
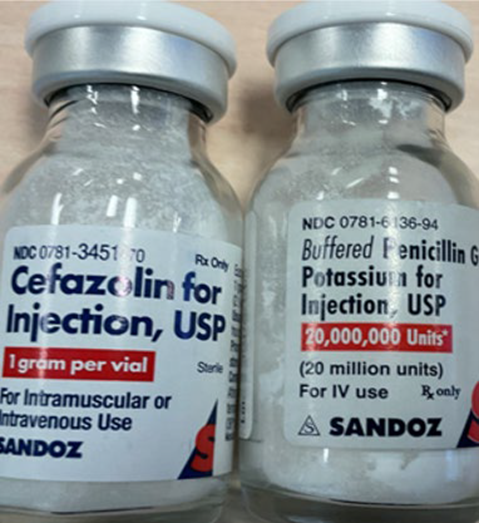
FOR IMMEDIATE RELEASE – Princeton, NJ – July 15, 2025 – Sandoz, Inc. (“Sandoz”) is initiating a voluntary nationwide recall expansion of one additional lot of Cefazolin for Injection, USP, 1 gram per vial. The lot is being recalled due to a customer complaint indicating that four (4) vials incorrectly labelled as Penicillin G Potassium for Injection, USP, 20 million Units were included in cartons (25 vials per carton) of Cefazolin for Injection, USP 1 gram per vial product. Sandoz has confirmed that the vials incorrectly labelled as Penicillin G Potassium for Injection contain Cefazolin for Injection, USP, 1 gram per vial.
Risk Statement: There is a reasonable probability that the inadvertent administration of cefazolin injection following dosing recommendation of penicillin G potassium injection due to mislabeling may pose serious and potentially life-threatening adverse health consequences, including lack of efficacy leading to less than optimal treatment of severe infections, antibiotic resistance, adverse reactions, severe allergic reactions (e.g., anaphylaxis), drug interactions, and delayed recovery.
To date, Sandoz has not received any reports of adverse events or injuries related to the product mislabeling. Sandoz has received a complaint of administration of the incorrectly labelled product to a patient.
Lots impacted by the voluntary recall and its expansion:
| Product Name | Vial NDC | Carton NDC | Lot Number | Expiration Date | Manufacturer | Distributor |
| Cefazolin for Injection, USP (25 by 1g vials) |
0781-3451-70 | 0781-3451-96 | PG4360 | 2027-NOV | Sandoz GmbH | Sandoz Inc |
| Penicillin G Potassium for Injection, USP | 0781-6136-94 | N/A | PG4360 | 2027-NOV | Sandoz GmbH | Sandoz Inc |
| Cefazolin for Injection, USP (25 by 1g vials) |
0781-3451-70 | 0781-3451-96 | PG4362 | 2027-NOV | Sandoz GmbH | Sandoz Inc |
| Penicillin G Potassium for Injection, USP | 0781-6136-94 | N/A | PG4362 | 2027-NOV | Sandoz GmbH | Sandoz Inc |
Cefazolin for Injection USP is used for the treatment of infections caused by certain bacteria in many different parts of the body including the treatment of pneumonia. Cefazolin for Injection USP can also be used to prevent infections, before and after surgery. Antibacterial drugs like Cefazolin for Injection USP treat only bacterial infections. They do not treat viral infections. Cefazolin for Injection USP is indicated for adult, elderly, pediatric patients, including newborn term infants.
Penicillin G Potassium for Injection is indicated in the treatment of certain serious infections including septicemia, skin and wound infections. It is also approved for the treatment of diphtheria, community-acquired pneumonia, peritonitis, meningitis/brain abscesses, osteomyelitis, infections of the genital tract, anthrax, tetanus, gas gangrene, listeriosis, pasteurellosis, rat bite fever, fusospirochetes, actinomycosis, complications in gonorrhea and syphilis and Lyme. To reduce the development of drug-resistant bacteria and maintain effectiveness of Penicillin G Potassium for Injection, USP and other antibacterial drugs, Penicillin G Potassium for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. Penicillin G Potassium for Injection is indicated for use in adults, adolescents, children, pediatric, newborn infants and preterm infants.
Although both Cefazolin and Penicillin G Potassium belong to the beta-lactam group of antibiotics, they are indicated for different types of infections, and the spectrum of susceptible organisms also differs. Additionally, while the patient populations overlap, each medicine has specific on-label distinct groups, and the dosing regimens may differ, as well.
Sandoz is notifying its customers by letter and is arranging for return of the recalled product. The product being recalled was shipped to select wholesalers for further distribution nationwide. Healthcare providers and customers who have this product should immediately stop use of this lot only and contact Sedgwick, the Sandoz Reverse Distributor, directly by phone at 1-844-265-7409 or by email at Sandoz5615@sedgwick.com.
For questions about the recall process, please call Sedgwick at 1-844-265-7409 between the hours of 8:00 AM to 5:00 PM Monday – Friday (EST).
Please report any adverse reactions by calling Sandoz at 1-800-525-8747. Customer service agents are available from 8:30 AM to 5:00 PM (EST), Monday-Friday, except on national holidays.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Note: The photos represent images of Lot PG4360. Potential mislabeling of Lot PG4362 would appear in a similar manner, except for the lot number printed on the packaging, which would read PG4362.
DISCLAIMER
This Media Release contains forward-looking statements, which offer no guarantee with regard to future performance. These statements are made on the basis of management’s views and assumptions regarding future events and business performance at the time the statements are made. They are subject to risks and uncertainties including, but not confined to, future global economic conditions, exchange rates, legal provisions, market conditions, activities by competitors and other factors outside of the control of Sandoz. Should one or more of these risks or uncertainties materialize or should underlying assumptions prove incorrect, actual outcomes may vary materially from those forecasted or expected. Each forward-looking statement speaks only as of the date of the particular statement, and Sandoz undertakes no obligation to publicly revise any forward-looking statements, except as required by law.
ABOUT SANDOZ
Sandoz (SIX: SDZ; OTCQX: SDZNY) is the global leader in generic and biosimilar medicines, with a growth strategy driven by its Purpose: pioneering access for patients. More than 20,000 people of 100 nationalities work together to ensure 900 million patient treatments are provided by Sandoz, generating substantial global healthcare savings and an even larger social impact. Its leading portfolio of approximately 1,300 products addresses diseases from the common cold to cancer. Headquartered in Basel, Switzerland, Sandoz traces its heritage back to 1886. Its history of breakthroughs includes Calcium Sandoz in 1929, the world’s first oral penicillin in 1951, and the world’s first biosimilar in 2006. In 2024, Sandoz recorded net sales of USD 10.4 billion
Company Contact Information
Consumers: Sedgwick, the Sandoz Reverse Distributor
1-844-265-7409; email at Sandoz5615@sedgwick.com
Media: Jeanne LaCour, Vicki Crafton
1-609-955-2339, 1-201-213-6338
Product Photos
If you have any further questions, please contact your Provider Services Representative, or call our Customer Service Department at 1-888-936-PLAN (7526). You may also visit https://www.hpsj.com/alerts/ for online access to the documents shared. The most recent information about Health Plan and our services is always available on our website www.hpsj-mvhp.org