Chantix Products Recall – IMMEDIATE ACTION NEEDED
| Date: | July 28, 2021 |
| To: | Health Plan of San Joaquin (HPSJ) Pharmacies and Providers |
| From: | HPSJ Pharmacy Department |
| Subject: | Chantix Products Recall – IMMEDIATE ACTION NEEDED |
| Business: | Medi-Cal Managed Care |
We are sending this alert because one or more of your patient(s) may have filled a prescription for Chantix Tablets within the last six months (180 days).
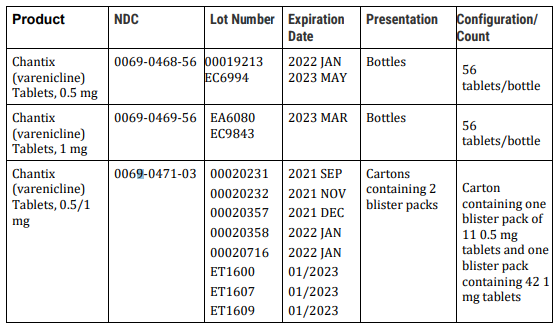
Recall: Pfizer is voluntarily recalling two lots of Chantix 0.5mg Tablets, two lots of Chantix 1 mg Tablets, and eight lots of a Chantix kit of 0.5mg/1 mg Tablets to the patient (consumer/user) level due to the presence of a nitrosamine, N-nitroso-varenicline, above the Pfizer established Acceptable Daily Intake (ADI) level.
Long-term ingestion of N-nitroso-varenicline may be associated with a theoretical potential
increased cancer risk in humans, but there is no immediate risk to patients taking this medication. The health benefits of stopping smoking outweigh the theoretical potential cancer risk from the nitrosamine impurity in varenicline.
HPSJ is now contacting all pharmacies and providers with HPSJ members who have filled a prescription for Chantix within the last six months (180 days).
This table shows the products subject to recall.
If you have questions, please contact our HPSJ Customer Service team at 1.888.936.PLAN (7526).

