Formulary Update Effective 8/3/2020
| Date: | May 22, 2020 |
| To: | Health Plan of San Joaquin (HPSJ) Physicians, Providers, and Pharmacies |
| From: | HPSJ Pharmacy and Therapeutics Committee |
| Subject: | Formulary Update |
| Business: | Medi-Cal |
Effective August 3, 2020, the Pharmacy and Therapeutics Committee has approved the following changes.
Additions to the Formulary:
1. Dulaglutide (Trulicity) 0.75 mg/0.5 mL, 1.5mg/0.5ml pen: PA required. Reserved for an inadequate response to 3 months of compliant use of dose-optimized Metformin with Jardiance OR Metformin with Steglatro (unless intolerant or contraindicated) with A1c <10%. A trial of Metformin ER is required if intolerance is GI-related.
2. Insulin Regular, Human (Novolin R) 100 units/ml FlexPen: Limited to 15 ml per 30 days.
3. Prucalopride Succinate (Motegrity) 1mg, 2mg tablet: PA required. Reserved for patients with Idiopathic Chronic Constipation, with treatment failure of properly titrated and regularly scheduled dosing of polyethylene glycol for 2 months (as evidenced by prescription history fills) AND two of the following: bisacodyl, senna, psyllium, magnesium citrate or hydroxide. Limited to 30 capsules per 30 days.
4. Naldemedine Tosylate (Symproic) 0.2 mg tablet: PA required. Reserved for patients with opioid-induced constipation with chronic noncancer pain and treatment failure of dose-optimized, regularly scheduled polyethylene glycol for 2 months (as evidenced by prescription history fills) AND two of the following: bisacodyl, senna, magnesium citrate or hydroxide. Limited to 30 capsules per 30 days.
5. Entacapone (Comtan) 200mg tablet: Limited to 8 tablets per day.
6. Istradefylline (Nourianz) 20mg, 40mg tablet: PA required. Reserved for patients experiencing at least 2 hours of “off” time per day despite treatment. Patients must have tried and failed treatment with dopamine agonists and COMPT inhibitors for “off” symptoms for at least four weeks. Limited to 30 tablets per 30 days.
Formulary Status Changes:
1. Semaglutide (Ozempic) 0.25-0.5mg dose pen, 1mg dose pen: PA required. Reserved for an inadequate response to 3 months of compliant use of dose-optimized Metformin with Jardiance OR Metformin with Steglatro (unless intolerant or contraindicated) with A1c <10%. A trial of Metformin ER is required if intolerance is GI-related.
2. Liraglutide (Victoza) 2-pak 18mg/3ml pen, 3-pak 18mg/3ml pen: PA required. Reserved for an inadequate response to 3 months of compliant use of dose-optimized Metformin with Jardiance OR Metformin with Steglatro (unless intolerant or contraindicated) with A1c <10%. A trial of Metformin ER is required if intolerance is GI-related.
3. Exenatide (Byetta) 5mcg, 10mcg dose pen, (Bydureon) 2mg pen, (Bydureon BCise) 2mg Autoinjector: PA required. Reserved for an inadequate response to 3 months of compliant use of dose-optimized Metformin with Jardiance OR Metformin with Steglatro (unless intolerant or contraindicated) AND Ozempic, Victoza, or Trulicity with A1c <10%. A trial of Metformin ER is required if intolerance is GI-related.
4. For Psoriasis – Apremilast (Otezla) 30mg tablet, 28-day starter pack: PA required. Reserved for treatment failure to an adequate trial of oral DMARD. If patient is unable to tolerate one oral DMARD, a second oral DMARD must be tried. Must be prescribed by a dermatologist. Restricted to specialty pharmacy.
5. For Ulcerative Colitis – Ustekinumab (Stelara) 45mg/0.5ml, 90mg/ml, 130mg/26ml syringe/vial: PA required. Reserved for treatment failure to Mesalamine, Thiopurine,
Cyclosporine, and TNF inhibitor. Must be at least 18 years of age AND have a documented diagnosis of moderate to severe ulcerative colitis. Restricted to specialty pharmacy. Must be prescribed by a gastroenterologist.
6. For Ankylosing Spondylitis – Secukinumab (Cosentyx) 150mg/ml (300mg dose), 150mg/ml syringe/pen: PA required. Reserved for treatment failure to Adalimumab, Etanercept, or Infliximab. Restricted to specialty pharmacy. Must be initiated by a rheumatologist.
7. For Ankylosing Spondylitis – Ixekizumab (Taltz) 80mg/ml autoinjector, syringe: PA
required. Reserved for treatment failure to Adalimumab, Etanercept, or Infliximab. Restricted to specialty pharmacy. Must be initiated by a rheumatologist.
8. Sodium Oxybate (Xyrem) 500mg/ml solution: PA required. Reserved for use in narcolepsy in patients who have tried and failed modafinil and a methyphenidate-based or amphetamine-based stimulant OR use in narcolepsy with cataplexy who are treated by a specialist. Limited to 540 ml per 30 days. Must be prescribed by a specialist.
9. Methylnaltrexone (Relistor) 12mg/0.6ml, 8mg/0.4ml syringe, vial and 150mg tablet: Changed to non-formulary. Options are Naloxegol and Symproic.
Deletions from the Formulary:
The following products will be removed from the formulary as of August 3, 2020: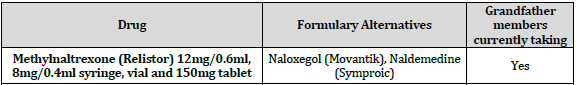
Health Plan of San Joaquin is dedicated to providing all members the best health care available in the most effective and efficient manner. We believe that this change in our Pharmacy Drug Benefit will not affect the quality of the care you provide.
You may contact our Customer Service Department with any questions or concerns Monday through Friday 8 a.m. to 5 p.m. at (209) 942-6320. Thank you for your continued support of Health Plan of San Joaquin.

