FDA Drug Recall Alert – Levetiracetam in 0.75% Sodium Chloride Injection 1,000 mg/100 mL
| Date: | March 18, 2025 |
| From: | Health Plan of San Joaquin/Mountain Valley Health Plan (“Health Plan”) |
| To: | Health Plan Practitioners, Facilities, and Hospitals |
| Type: | Informational |
| Subject: | FDA Drug Recall Alert – Levetiracetam in 0.75% Sodium Chloride Injection 1,000 mg/100 mL |
| Business: | Medi-Cal Managed Care |
On March 13, 2025, the Food and Drug Administration (FDA) released a recall announcement on Levetiracetam in 0.75% Sodium Chloride Injection 1,000 mg/100 mL. This is a courtesy notification only. You may or may not have administered the recalled medication. Please disregard if you have no relevance to this alert. For the complete details regarding this recall announcement, please visit the following link: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/dr-reddys-issues-nationwide-recall-levetiracetam-075-sodium-chloride-injection-1000-mg100-ml-us-due?utm_medium=email&utm_source=govdelivery
Dr. Reddy’s issues a Nationwide Recall of Levetiracetam in 0.75% Sodium Chloride Injection 1,000 mg/100 mL, in the U.S., Due to Mislabeling of Infusion Bag.
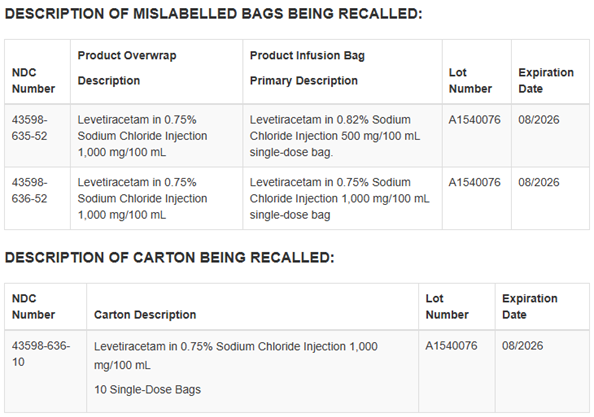
Company Announcement: Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; along with its subsidiaries together referred to as “Dr. Reddy’s”), is recalling one Batch/Lot No: A1540076 of Levetiracetam in 0.75% Sodium Chloride Injection, 1,000 mg/100 mL (10 mg/mL) single-dose infusion bags to the consumer level, in the United States.
The product is being recalled because the infusion bag is incorrectly labeled as Levetiracetam in 0.82% Sodium Chloride Injection 500 mg/100 mL single-dose bag, while the aluminum overwrap packaging correctly identifies the product as Levetiracetam in 0.75% Sodium Chloride Injection 1,000 mg/100 mL.
Risk Statement: Patients who are administered the mislabeled product will likely experience adverse events. Because the infusion bag is labelled as 500 mg/100 mL but actually contains 1,000 mg/100 mL dose, the patient could receive double the dose of intravenous levetiracetam than intended which could lead to immediate and serious side effects including hypersensitivity reactions, liver injury, hematological toxicity, somnolence, fatigue, dizziness, coordination difficulties, agitation, aggression, depressed level of consciousness, respiratory depression, and coma. Patients receiving high doses of levetiracetam by rapid intravenous infusion for the treatment of status epilepticus would be most at risk for severe adverse events. Dr. Reddy’s has not received any reports of adverse events related to this recall.
Levetiracetam in 0.75% Sodium Chloride Injection, 1,000 mg/100 mL (10 mg/mL) and Levetiracetam in 0.82% Sodium Chloride Injection, 500 mg/100 mL (5mg/mL) are both indicated for adjunct therapy in adults (≥16 years of age) with the following seizure types when oral administration is temporarily not feasible:
- Partial onset seizures
- Myoclonic seizures in patients with juvenile myoclonic epilepsy
- Primary generalized tonic-clonic seizures
Each product is packaged in single-dose infusion bags with an aluminum overwrap, 10 single-dose bags packed in a carton. Identification information such as lot number, expiration date and NDC is presented in the table below. The batch was distributed nationwide between November 4, 2024, and November 6, 2024, to wholesalers.
Dr. Reddy’s Laboratories Inc is notifying its distributors and customers to arrange for return of any recalled product. Wholesalers, distributors, hospitals, and pharmacies with an existing inventory of the lot being recalled, should stop use and distribution and quarantine the product immediately for return/replacement of all recalled products. Wholesalers, distributors, and pharmacies that have further distributed the recalled product should notify any accounts or additional locations which may have received the recalled product from them. For instructions on returning product or additional assistance, call Inmar at 1-877-645-1584 between the hours of 9 a.m. to 5 p.m. ET, Monday through Friday.
Consumers with questions regarding this recall can contact Dr. Reddy’s Medical Information Call Center at 1-888-375-3784 (1-888-DRL-DRUG) between the hours of 8 a.m. to 8 p.m. ET, Monday through Friday. Consumers should contact their healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report found here: https://www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Regular Mail or Fax: https://www.fda.gov/safety/medical-product-safety-information/medwatch-forms-fda-safety-reporting or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
Consumers: Dr. Reddy’s Medical Information Call Center 1-888-375-3784 (1-888-DRL-DRUG)
If you have any further questions, please contact your Provider Services Representative, or call our Customer Service Department at 1-888-936-PLAN (7526). You may also visit https://www.hpsj.com/alerts/ for online access to the documents shared. The most recent information about Health Plan and our services is always available on our website www.hpsj-mvhp.org