FDA Drug Recall Alert – Lactated Ringer’s Injection USP 1000 mL/0.9% Sodium Chloride Injection USP 1000 mL
| Date: | August 26, 2025 |
| From: | Health Plan of San Joaquin/Mountain Valley Health Plan (“Health Plan”) |
| To: | Health Plan Practitioners, Facilities, and Hospitals |
| Type: | Informational/Educational |
| Subject: | FDA Drug Recall Alert – Lactated Ringer’s Injection USP 1000 mL/0.9% Sodium Chloride Injection USP 1000 mL |
| Business: | Medi-Cal Managed Care |
On August 19, 2025, the Food and Drug Administration (FDA) released a recall announcement on Lactated Ringer’s Injection USP 1000 mL/0.9% Sodium Chloride Injection USP 1000 mL.
This is for informational purposes only. You may or may not have administered the medication. Please disregard if you have not been affected by this recall.
For the complete details regarding this recall announcement, please visit the following web link: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/b-braun-medical-issues-voluntary-nationwide-recall-lactated-ringers-injection-usp-1000-ml-and-09?utm_medium=email&utm_source=govdelivery
Braun Medical Issues Voluntary Nationwide Recall of Lactated Ringer’s Injection USP 1000 mL and 0.9% Sodium Chloride Injection USP 1000 mL Due to the Presence of Particulate Matter
Summary
Company Announcement Date: August 19, 2025
FDA Publish Date: August 19, 2025
Product Type: Drugs
Reason for Announcement: Due to the presence of particulate matter.
Company Name: B. Braun Medical, Inc.
Brand Name: B. Braun
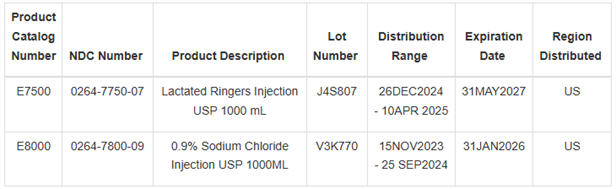
Product Description: Lactated Ringer’s Injection USP 1000 mL/0.9% Sodium Chloride Injection USP 1000 mL
Company Announcement
For Immediate Release – BETHLEHEM, PA – August 19, 2025 – B. Braun Medical Inc. (B. Braun) is voluntarily recalling two lots of Lactated Ringers Injection USP 1000 mL, and 0.9% Sodium Chloride Injection USP 1000 mL to the hospital level due to the presence of particulate matter inside the container.
B. Braun has identified through complaints the potential for the product to contain particulate matter in solution. To date there have been no reports of serious injury, death or other adverse events associated with this issue. If the particulate matter is observed before use, a minor delay could occur while obtaining a replacement product. If the particulate matter is loose and the container is used on a patient, there is a potential for the particulate to be infused into the circulatory system. This could lead to patient harm that may require additional medical intervention and/or lead to permanent impairment or death.
The product has a reasonable probability of causing pulmonary emboli (blockage in pulmonary blood vessels), occlusions of other blood vessels (which can lead to tissue death and possible organ damage), and/or phlebitis (inflammation of the walls of veins, which may lead to clotting). Systemically, foreign particles infused intravenously can cause systemic activation of the immune system, organ dysfunction, and hemolysis (breakdown of blood cells). To date there have been no reports of serious injury, death or other adverse events associated with this issue.
0.9% Sodium Chloride Injection USP is indicated for extracellular fluid replacement, treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion. Lactated Ringers Injection USP 1000 mL solution is indicated for use in adults and pediatric patients as a source of electrolytes and water for hydration. These products are packaged in boxes of 12 each. Additional details on the affected products are as follows:
These products were distributed nationwide via distributors.
B. Braun is notifying its distributors and customers by certified mail and is arranging for return. of all recalled products. Distributors that have affected product which is being recalled should determine their current inventory of the affected items within inventory of their facility, cease use and distribution and quarantine product subject to recall. Affected product should not be destroyed.
Customers who have questions about this recall should contact our B. Braun’s Recalls Department at 844-903-6417 between 9 AM and 5 PM EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
- Complete and submit the report online: fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About B. Braun
B. Braun Medical Inc., a leader in infusion therapy and pain management, develops, manufactures, and markets medical products and services to the healthcare industry. Other key product areas include nutrition, pharmacy admixture and dialysis. The company is committed to eliminating preventable treatment errors and enhancing patient, clinician and environmental safety. B. Braun Medical is headquartered in Bethlehem, Pennsylvania and is part of the B. Braun Group of Companies in the U.S., which includes B. Braun Interventional Systems, Aesculap® and CAPS®.
Globally, the B. Braun Group of Companies employs more than 64,000 employees in 64 countries. Guided by its Sharing Expertise® philosophy, B. Braun continuously exchanges knowledge with customers, partners and clinicians to address the critical issues of improving care and lowering costs.
To learn more about B. Braun Medical, explore our website: https://www.bbraunusa.com/en.html.
Company Contact Information:
Consumers: B. Braun’s Recalls Department at 844-903-6417
Media: 484-523-9801; email at allison.longenhagen@bbraunusa.com
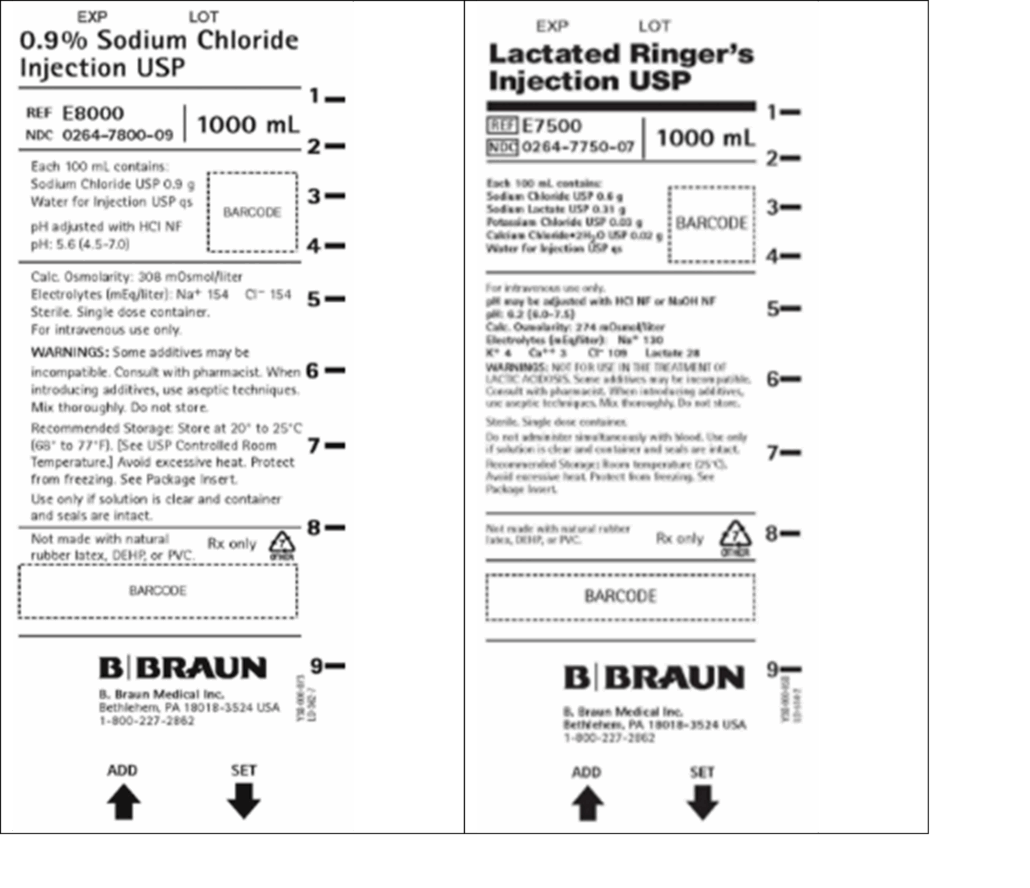
Product Photos
If you have any further questions, please contact your Provider Services Representative, or call our Customer Service Department at 1-888-936-PLAN (7526). You may also visit https://www.hpsj.com/alerts/ for online access to the documents shared. The most recent information about Health Plan and our services is always available on our website www.hpsj-mvhp.org