Enoxaparin Products Recall – IMMEDIATE ACTION NEEDED
| Date: | February 8, 2021 |
| To: | Health Plan of San Joaquin (HPSJ) Pharmacies and Providers |
| From: | HPSJ Pharmacy Department |
| Subject: | Enoxaparin Products Recall – IMMEDIATE ACTION NEEDED |
| Business: | Medi-Cal |
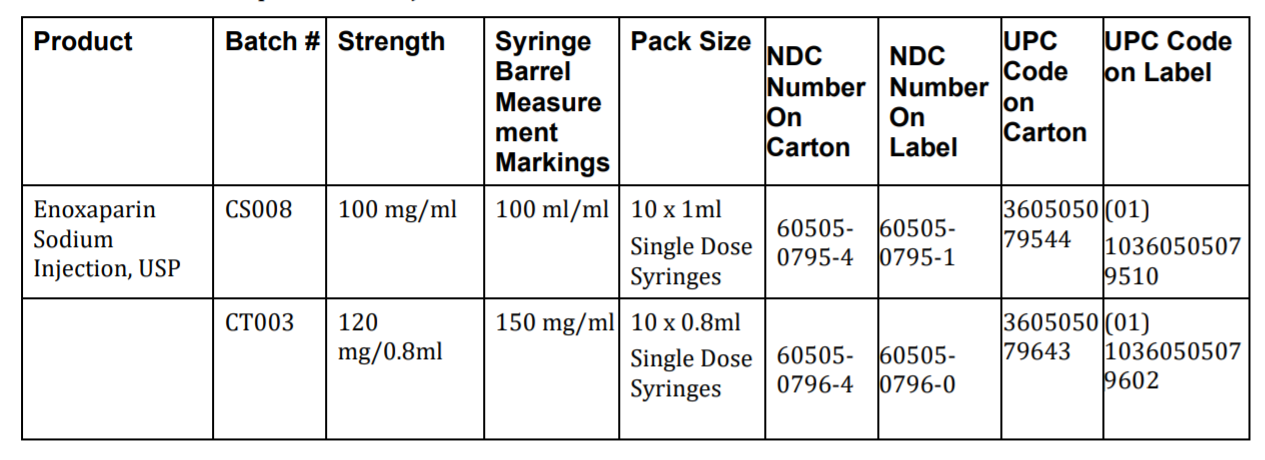
We are sending this alert because one or more of your patient(s) may have filled a prescription for Enoxaparin Sodium Injection within the last twelve months (365 days). Recall: Apotex Corp is voluntarily recalling two (2) batches of Enoxaparin Sodium Injection, USP to consumer level due to a packaging error resulting in some syringes barrels containing 150 mg/mL markings (corresponding to 120 mg/0.8mL strength) instead of 100 mg/mL markings (corresponding to 100 mg/mL strength) on the syringe barrel and vice versa.
- Incorrect syringe barrel marking could lead to miscalculation and inaccurate dose
administration to patients. - Accidental over dosage following administration of enoxaparin sodium injection may lead to bleeding complications.
- If the dose administered is less than prescribed, the patient may be subject to
developing some blood clotting conditions.
HPSJ is now contacting all HPSJ members who have filled a prescription for Enoxaparin within the last twelve months (365 days). We are recommending they contact their pharmacy to check to see if their medication is part of the recall. This table shows the product subject to recall.
 If you have questions, please contact our HPSJ Customer Service team (Monday-Friday, 8 a.m. – 6 p.m. PST), at 209-942-6320.
If you have questions, please contact our HPSJ Customer Service team (Monday-Friday, 8 a.m. – 6 p.m. PST), at 209-942-6320.

