Improving the Quality of Care: Risks Associated with Use of Gabapentin

Learning Objectives:
- Review the U.S. Food and Drug Administration (FDA) approved indications for gabapentinoids (gabapentin and pregabalin).
- Describe potential risks associated with combining gabapentin with opioids.
- Summarize best practices for responsible prescribing of gabapentin.
Key Points:
- Recent restrictions on opioid prescribing have led clinicians to utilize alternative or complementary approaches to pain management, especially for patients with chronic pain. Gabapentin is often prescribed concomitantly with opioids to fulfill this need.
- Gabapentin is FDA approved to treat postherpetic neuralgia in adults. Gabapentin is also FDA approved as adjunctive therapy in the treatment of partial onset seizures, with or without secondary generalization, in adults or pediatric patients three years of age or older with epilepsy. There is limited evidence supporting the efficacy of gabapentin for off-label uses.
- On December 19, 2019, the FDA announced that serious, life-threatening, and fatal respiratory depression has been reported with use of gabapentin and pregabalin. Most cases occurred in association with co-administered central nervous system (CNS) depressants, especially opioids, in the setting of underlying respiratory impairment, or in the elderly.
- While gabapentin is not currently scheduled under the Controlled Substances Act of 1970, a number of states are implementing regulatory approaches to mitigate diversion and abuse of gabapentin. Prescribers should know of the abuse liability and associated effects of gabapentin, recognize the current diversion of gabapentin, and dispense gabapentin judiciously.
- Use of gabapentin in the Medi-Cal population rose from 60.4 paid claims per 1,000 eligible beneficiaries to 131.7 paid claims per 1,000 eligible beneficiaries, an increase of 118% between 2009 through 2018.
- Within a subset of continuously eligible Medi-Cal fee-for-service beneficiaries, only 13% of beneficiaries (n = 505) had an FDA-approved diagnosis for gabapentin during the measurement year and almost half (44%; n = 1,769) had concomitant use of gabapentin and opioid medications.
- Before prescribing gabapentin with opioids, the potential risks and benefits should be considered and discussed with patients, including the increased potential for life-threatening respiratory depression. When concomitant use of gabapentin and opioid medications is deemed medically necessary, a prescription for naloxone or another drug approved by the FDA for the complete or partial reversal of opioid-induced respiratory depression should be offered to patients and/or caregivers.
Background
Gabapentinoids (gabapentin and pregabalin) are frequently prescribed with opioids for their opioid-sparing and adjuvant analgesic effects. However, both gabentin and pregabalin are FDA-approved for limited indications. Gabapentin is FDA-approved to treat neuralgia in adults. Gabapentin is also FDA approved as adjunctive therapy in the treatment of partial onset seizures, with or without secondary generalization, in adults and pediatric patients three years of age or older with epilepsy.1 Pregabalin is FDA-approved for neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, adjunctive therapy for adult patients with partial onset seizures, fibromyalgia, and neuropathic pain associated with spinal cord injury.2 The mechanism of action for both of these agents has not been fully elucidated.
Despite these limited indications, use of these drugs has tripled during the past 15 years, with gabapentin ranked as the 10th most commonly prescribed drug in the United States in 2017.3,4 This increase likely reflects off-label use of gabapentin for managing pain conditions, in part to reduce or avoid opioid use. In the Medi-Cal fee-for-service program, pregabalin is available only with an approved Treatment Authorization Request (TAR) and gabapentin is on the Medi-Cal fee-for-service List of Contract Drugs without any additional restrictions.
A Journal of American Medical Association (JAMA) Special Communication published in 2019 identified 362 publications on gabapentinoids and pain.5 Of these, 34 randomized, controlled trials for noncancer, non-FDA approved pain conditions were identified and reviewed. The duration of most of the trials was 4 – 12 weeks. No evidence was found to support the use of gabapentinoids for low back pain or radiculopathy. In addition, no clear benefit was noted for the use of gabapentin to treat diabetic neuropathy, and either negative results or small differences in improvement were identified for nondiabetic neuropathies. For other pain syndromes, a questionable significant benefit was noted (1 point or less improvement on a pain scale of 0 to 10). The authors commented that there continues to be uncertainty as to whether efficacy for one type of neuropathic pain translates to efficacy for other types of neuropathic pain, regardless of suspected etiology.
On December 19, 2019, the FDA announced they are requiring new warnings be added to the prescribing information of gabapentinoids due to reports of serious, life-threatening, and fatal respiratory depression associated with the use of gabapentin and pregabalin.6 The FDA is also requiring drug manufacturers to conduct clinical trials to evaluate the abuse potential of gabapentinoids, particularly in combination with opioids, because misuse and abuse of these products together is increasing, and co-use may increase the risk of respiratory depression. To read the full safety announcement, which includes a review of cases from the FDA Adverse Event
Reporting System (FAERS) database and the medical literature, refer to the “FDA Drug Safety Communication: FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) when used with CNS depressants or in patients with lung problems” article found on the Drug Safety and Availability page of the FDA website.
Potential Misuse and Abuse of Gabapentin
Gabapentin potentiates the effect of opioids, and the combination increases the risk of respiratory depression and overdose. Recent reports suggest concomitant use of gabapentin and opioids might be an indicator of high-risk opioid misuse and could increase the risk of serious adverse events.6-13 A population-based case-control study among opioid users in Canada found a 49% increased risk of opioid-related death in opioid users taking concomitant gabapentin when compared with opioid use alone, even after adjustment for potential influences such as opioid dose.9 A moderate dose (900 to 1799 mg daily of gabapentin) or high dose (1800 mg or more daily of gabapentin) was associated with almost a 60% increase in the odds of opioid-related death when compared with the use of opioids alone.9
In a 2018 review by the Centers for Disease Control and Prevention (CDC) Enhanced State Opioid Overdose Surveillance (ESOOS) program, gabapentin use was associated with approximately 21.6% of opioid deaths, 8.1% of deaths involving illicit opioids, and 14.9% of deaths involving both prescription and illicit opioids, or one in five prescription opioid-only deaths.10,11 A 10-year (2005 – 2015) review of reports from the FAERS also identified abuserelated signals for gabapentin.12 Approximately 25% of all adverse event reports for gabapentin were deemed to be abuse-related adverse events. Signals for drug diversion, substance use, and substance abuse were noted for gabapentin. Compared to a negative control (duloxetine), gabapentin had over six times the odds of a co-report of drug abuse and drug withdrawal syndrome.12
While pregabalin is a Schedule V controlled substance, gabapentin is not currently scheduled under the Controlled Substances Act of 1970. However, recognition of gabapentin as an opportunistic drug of abuse has led to a number of states implementing regulatory approaches to mitigate diversion and abuse of gabapentin.13 Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia now classify gabapentin as a Schedule V controlled substance and numerous other states now mandate reporting of gabapentin to a prescription drug monitoring program or are reviewing legislation in order to label gabapentin as a Schedule V controlled substance.
Use of Gabapentin in the Medi-Cal Population
A retrospective cohort study was conducted to evaluate utilization of gabapentinoid medications in the Medi-Cal population. All paid pharmacy claims for gabapentinoid medications among Medi-Cal beneficiaries with dates of service from January 1, 2010, through October 31, 2019, were included. Due to low utilization of gabapentin enacarbil, only paid claims for gabapentin and pregabalin were included in the analysis.
Complete calendar year pharmacy claims data for 2010 – 2018 were controlled for Medi-Cal enrollment growth by calculating paid claims per 1,000 certified eligible Medi-Cal beneficiaries (Figure 1)
Figure 1. Gabapentinoid Utilization Trends in the Medi-Cal Population (Paid Claims per 1,000 Certified Eligible Medi-Cal Beneficiaries)
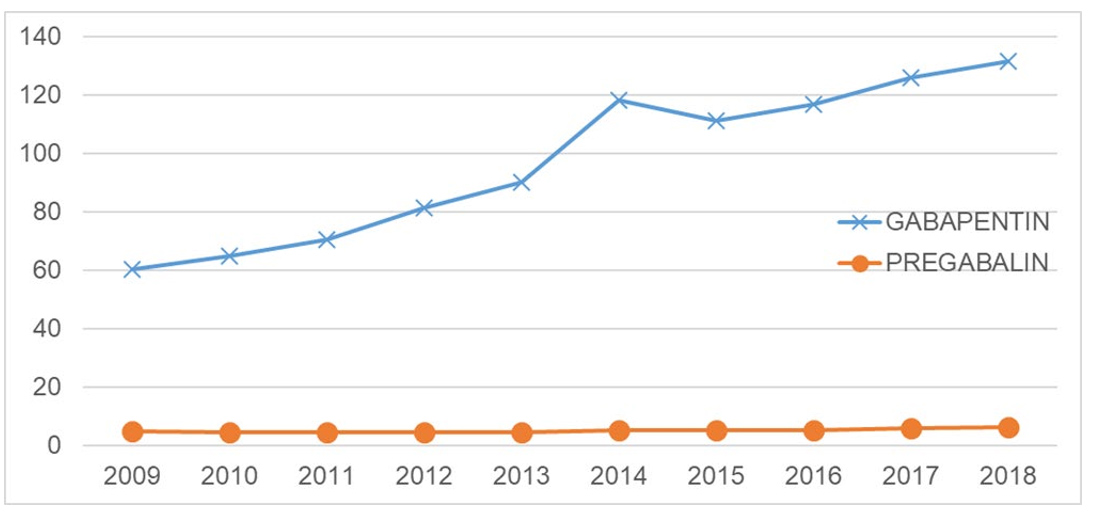
From 2009 through 2018, use of gabapentin in the Medi-Cal population rose from 60.4 paid claims per 1,000 eligible beneficiaries to 131.7 paid claims per 1,000 eligible beneficiaries, an increase of 118%. The overall percentage of Medi-Cal beneficiaries with at least one paid claim for gabapentin also increased by 107% during this same time period (1.4% utilization in 2009, compared with 2.9% utilization in the Medi-Cal population in 2018). While pregabalin use also increased during this same time period (4.9 paid claims per 1,000 eligible beneficiaries to 6.3 paid claims per 1,000 eligible beneficiaries), the increase was not as significant (an increase of 28%). Similarly, the overall percentage of Medi-Cal beneficiaries with at least one paid claim of pregabalin in the Medi-Cal population increased by 19% between 2009 through 2018, staying constant at 0.1% of the Medi-Cal population.
A review of paid claims for gabapentin during the last 12 months (dates of service from November 1, 2018, through October 31, 2019; the measurement year) showed a total of 371,086 Medi-Cal beneficiaries had at least one paid claim for gabapentin, with the vast majority (92.5%) enrolled in a Medi-Cal Managed Care Plan (MCP).
An additional evaluation was conducted on 4,002 beneficiaries who had at least one paid claim for gabapentin and were continuously eligible in the Medi-Cal fee-for-service program during the measurement year. A review of concomitant medications for these beneficiaries found that 44.2%
(n = 1,769) had a paid claim for an opioid medication within 30 days of a paid claim for gabapentin, and 10.3% (n = 414) had concomitant use of gabapentin, at least one opioid medication, and two additional CNS depressants, including benzodiazepines, skeletal muscle relaxants, other sleep drugs and tranquilizers (non-benzodiazepine), antipsychotic medications, and other selected psychotropic medications with CNS-depressant properties
Finally, medical claims were reviewed for these 4,002 beneficiaries in order to assess the potential off-label use of gabapentin in this population. A broad definition of FDA-approved primary and secondary ICD-10-CM diagnosis codes was used, including all neuralgia, seizures/epilepsy, peripheral neuropathy, fibromyalgia, and neuropathic pain. In order to capture any diagnosis codes not captured during the measurement year, medical claims were reviewed with dates of service from November 1, 2014, through October 31, 2019, for a full five years of medical claims. Even using generous criteria to assess FDA-approved diagnosis codes, only 12.6% of these beneficiaries (n = 505) had a diagnosis for an FDA-approved indication over the five-year period.
Conclusion/Discussion
The use of gabapentin for pain is intended to decrease the use of opioids; however, many patients are prescribed both classes of drugs concomitantly. Off-label use of gabapentinoids to manage pain has limited or questionable benefit but is associated with the risk of serious side effects including dizziness, somnolence, unsteadiness, euphoria, and respiratory depression. There are increasing reports of gabapentin being misused and abused for recreation, self-medication, and/or self-harm. Patients with a history of or current opioid misuse are at particular risk for adverse events, including opioid-related death.
Clinical Recommendations:
- Gabapentin prescribers should know of its abuse liability and associated effects, recognize the current diversion of gabapentin, and dispense judiciously.
- Where possible, limit prescribing of gabapentin to FDA-approved diagnoses at the recommended doses. In cases where off-label use is warranted, clinicians should start at the lowest dose possible and advise patients that the proposed use is off-label and that adverse effects, such as unusual dizziness and somnolence, are common.
- Before prescribing gabapentin with opioids, the potential risks and benefits should be considered and discussed with patients, including the increased potential for life-threatening respiratory depression. When concomitant use of gabapentin and opioid medications is deemed medically necessary, a prescription for naloxone or another drug approved by the FDA for the complete or partial reversal of opioid-induced respiratory depression should be offered to patients and/or caregivers.
- Any therapeutic trial of gabapentin in combination with an opioid medication should be closely monitored and discontinued if there is little or no perceived benefit.
- Due to the unpredictable pharmacokinetics and non-linear bioavailability of gabapentin, patients prescribed gabapentin should be closely monitored for adverse effects.
Potential strategies to minimize the risk of a serious interaction include cautious dose titration, dose adjustment with concomitant lung and kidney disease, and avoidance of additional concomitant CNS depressants. Cautious prescribing is advised for patients with respiratory disease and elderly patients.
References:
- Neurontin (package insert). New York, NY: Parke-Davis, Division of Pfizer, Inc.; 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_0 21129s046lbl.pdf. Accessed: September 9, 2019.
- Lyrica (package insert). New York, NY: Parke-Davis, Division of Pfizer, Inc.; 2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021446s036,022488s014lbl. pdf. Accessed: September 9, 2019.
- Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292-294. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5838608/. Accessed: September 9, 2019.
- IQVIA Institute. Medicine use and spending in the US: a review of 2017 and outlook to 2022. https://www.iqvia.com/Institute/Reports/Medicine-Use-And-Spending-In-The-UsReview-Of-2017-Outlook-To-2022. Published April 19, 2018. Accessed: September 9, 2019.
- Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med 2019;179(5):695-701.
- FDA Drug Safety Communication. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) when used with CNS depressants or in patients with lung problems. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warnsabout-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentinneurontin. Accessed: December 19, 2019.
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 015;172:487–8. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4864031/pdf/nihms781657.pdf. Accessed: September 9, 2019.
- Evoy KE, Morrison MD, Saklad SR. Abuse and Misuse of Pregabalin and Gabapentin. Drugs. 2017;77(4):403-426. doi:1007/s40265-017-0700-x
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. Available at: https://journals.plos.org/plosmedicine/article/file?id=10.1371/journal.pmed.1002396&type =printable. Accessed: September 9, 2019.
- Mattson CL, O’Donnell J, Kariisa M, et al. Opportunities to prevent overdose deaths involving prescription and illicit opioids, 11 states, July 2016-June 2017. MMWR Morb Mortal Wkly Rep 2018; 67:945-51. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/pdfs/mm6734a2-H.pdf. Accessed: September 9, 2019.
- Scholl L, Seth P, et al. Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. MMWR. 2019;67(5152);1419–1427. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/pdfs/mm675152e1-H.pdf. Accessed: September 9, 2019.
- Vickers-Smith R, Sun J, Charnigo RJ, et al. Gabapentin drug misuse signals: a pharmacovigilance assessment using the FDA adverse event reporting system. Drug Alcohol Depend. 2019 Nov 2:107709.
- Peckham AM, Ananickal MJ, and Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy. 2018 Aug 17;11:109-116. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6103607/pdf/rmhp-11-109.pdf. Accessed: September 9, 2019.

